The State of Balance or Normality That the Human Body Continuously Tries to Attain is Referred to as
An introduction to acid-base balance in health and disease
Normal cell metabolism depends on the maintenance of blood pH within very narrow limits (7.35-7.45).
Even relatively mild excursions outside this normal pH range can have deleterious effects, including reduced oxygen delivery to tissues, electrolyte disturbances and changes in heart muscle contractility; survival is rare if blood pH falls below 6.8 or rises above 7.8.
The problem for the body is that normal metabolism is associated with continuous production of hydrogen ions (H+) and carbon dioxide (CO2), both of which tend to reduce pH. The mechanism which overcomes this problem and serves to maintain normal blood pH (i.e. preserve acid-base homeostasis) is a complex synergy of action involving chemical buffers in blood, the red cells (erythrocytes), which circulate in blood, and the function of three organs: lungs, kidneys and brain.
Before explaining how these five elements contribute to the overall maintenance of blood pH, it would be helpful to quickly review some basic concepts.
What is an acid, what is a base and what is pH?
An acid is a substance which releases hydrogen ions (H+) on dissociation in solution.
For example: Hydrochloric acid (HCl) dissociates to hydrogen ions and chloride ions
HCl![]() H+ + Cl-
H+ + Cl-
Carbonic acid (H2CO3) dissociates to hydrogen ions and bicarbonate ions
H2CO3 ![]() H+ + HCO3 –
H+ + HCO3 –
We distinguish between strong acids like hydrochloric acid and weak acids like carbonic acid. The difference is that strong acids dissociate more than weak acids. Consequently the hydrogen ion concentration of a strong acid is much higher than that of a weak acid.
A base is a substance which in solution accepts hydrogen ions.
For example, the base bicarbonate (HCO3 –) accepts hydrogen ions to form carbonic acid:
HCO3 – + H+ ![]() H2CO3
H2CO3
pH is a scale of 0-14 of acidity and alkalinity. Pure water has a pH of 7 and is neutral (neither acidic nor alkaline). pH above 7 is alkaline and below 7 acidic. Thus the pH of blood (7.35-7.45) is slightly alkaline although in clinical medicine the term alkalosis is, perhaps confusingly, reserved for blood pH greater than 7.45 and the term acidosis is reserved for blood pH less than 7.35.
pH is a measure of hydrogen ion concentration (H+). The two are related according to the following equation:
pH = - log10 [H+]
where [H+] is the concentration of hydrogen ions in moles per liter (mol/L)
From this equation
pH 7.4 = H+ concentration of 40 nmol/L
pH 7.0 = H+ concentration of 100 nmol/L
pH 6.0 = H+ concentration of 1000 nmol/L
It is clear that:
- the two parameters change inversely; as hydrogen ion concentration increases, pH falls
- due to the logarithmic relationship, a large change in hydrogen ion concentration is actually a small change in pH. For example, doubling the hydrogen ion concentration causes pH to fall by just 0.3
What is a buffer? – the bicarbonate buffer system
Buffers are chemicals in solution which minimize the change in pH which occurs when acids are added by 'mopping up' hydrogen ions. A buffer is a solution of a weak acid and its conjugate base. In blood, the principle buffer system is the weak acid, carbonic acid (H2CO3) and its conjugate base, bicarbonate (HCO3 –). To explain how this system minimizes changes in pH, suppose we add a strong acid, e.g. HCl, to the bicarbonate buffer:
The acid will dissociate, releasing hydrogen ions:
HCl ![]() H+ + Cl–
H+ + Cl–
The bicarbonate buffer then 'absorbs' the hydrogen ions, forming carbonic acid in the process:
HCO3 – + H+ ![]() H2CO3 (carbonic acid)
H2CO3 (carbonic acid)
The important point is that because the hydrogen ions from HCl have been incorporated into the weak carbonic acid, which does not dissociate as easily, the total number of hydrogen ions in solution and therefore the pH do not change as much as would have occurred in the absence of the buffer.
Although a buffer greatly minimizes pH change, it does not eliminate it because even a weak acid (like carbonic acid) dissociates to some extent. The pH of a buffer solution is a function of the relative concentrations of the weak acid and its conjugate base.
pH = 6.1 + log ([HCO3 –] / [H2CO3])
Where [HCO3 –] = concentration of bicarbonate
[H2CO 3] = concentration of carbonic acid
This relationship, known as the Henderson-Hasselbalch equation, shows that pH is governed by the ratio of base (HCO3 –) concentration to acid (H2CO3) concentration.
As hydrogen ions are added to the bicarbonate buffer:
H+ + HCO3 –![]() H2CO3
H2CO3
bicarbonate (base) is consumed (concentration decreases) and carbonic acid is produced (concentration increases). If hydrogen ions continue to be added, all bicarbonate would eventually be consumed (converted to carbonic acid) and there would be no buffering effect – pH would then fall sharply if more acid were added.
However, if carbonic acid could be continuously removed from the system and bicarbonate constantly regenerated, then the buffering capacity and therefore pH could be maintained despite continued addition of hydrogen ions.
As will become clear with more detail of the physiology of acid-base balance, that is, in effect, what happens in the body. In essence, the lungs ensure removal of carbonic acid (as carbon dioxide) and the kidneys ensure continuous regeneration of bicarbonate.
This role of the lungs is dependent on a singular characteristic of the bicarbonate buffering system and that is the ability of carbonic acid to be converted to carbon dioxide and water.
The following equation outlines the relationship of all elements of the bicarbonate buffering system as it operates in the body
H+ + HCO3 –![]() H2CO3
H2CO3![]() H2O + CO2
H2O + CO2
It is important to note that the reactions are reversible. Direction is dependent on the relative concentration of each element. So that, for example, a rise in carbon dioxide concentration forces reaction to the left with increased formation of carbonic acid and ultimately hydrogen ions.
This explains the acidic potential of carbon dioxide and brings us to the important contribution that the lungs and red cells make to overall acid-base balance.
Lung function, transport of CO2 and acid-base balance
A constant amount of CO2 in blood, essential for normal acid-base balance, reflects a balance between that produced as a result of tissue cell metabolism and that excreted by the lungs in expired air.
By varying the rate at which carbon dioxide is excreted, the lungs regulate the carbon dioxide content of blood. The sequence of events from carbon dioxide production in the tissues to elimination in expired air is described in Fig. 1. Carbon dioxide diffuses out of tissue cells to surrounding capillary blood (Fig. 1a). A small proportion dissolves in blood plasma and is transported to the lungs unchanged.
But most diffuses into red cells where it combines with water to form carbonic acid. The acid dissociates with production of hydrogen ions and bicarbonate. Hydrogen ions combine with deoxygenated hemoglobin (hemoglobin is acting as a buffer here), preventing a dangerous fall in cellular pH, and bicarbonate diffuses along a concentration gradient from red cell to plasma.
Thus most of the carbon dioxide produced in the tissues is transported to the lungs as bicarbonate in blood plasma.
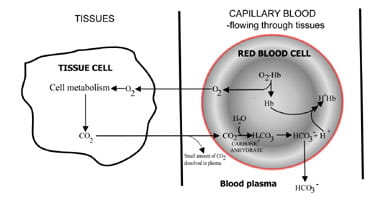
Fig. 1a. CO2 produced in tissues converted to bicarbonate for transport to lungs.
|
|
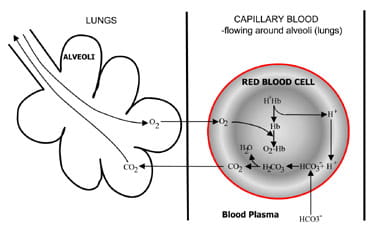
Fig. 1b. At the lungs bicarbonate converted back to CO2 and eliminated by the lungs.
At the alveoli in the lungs the process is reversed (Fig. 1b). Hydrogen ions are displaced from hemoglobin as it takes up oxygen from inspired air. The hydrogen ions are now buffered by bicarbonate which diffuses from plasma back into red cell, and carbonic acid is formed. As the concentration of this rises, it is converted to water and carbon dioxide. Finally, carbon dioxide diffuses down a concentration gradient from red cell to alveoli for excretion in expired air.
Respiratory chemoreceptors in the brain stem respond to changes in the concentration of carbon dioxide in blood, causing increased ventilation (breathing) if carbon dioxide concentration rises and decreased ventilation if carbon dioxide falls.
Kidneys and acid-base balance
Normal cellular metabolism results in continuous production of hydrogen ions. We have seen that by combining with these hydrogen ions, the bicarbonate buffer in blood minimizes their effect. However, buffering is only useful in the short term, and ultimately hydrogen ions have to be removed from the body. Furthermore, it is important that the bicarbonate that is used to buffer hydrogen ions is continuously replaced.
These two tasks, elimination of hydrogen ions and regeneration of bicarbonate, are accomplished by the kidneys. Renal tubule cells are rich in the enzyme carbonic anhydrase, which facilitates formation of carbonic acid from carbon dioxide and water. Carbonic acid dissociates to bicarbonate and hydrogen ions. The bicarbonate is reabsorbed into blood and the hydrogen ions pass into the lumen of the tubule and are eliminated from the body in urine.
This urinary elimination is dependent on the presence in urine of buffers, principally phosphate and ammonia ions.
DISTURBANCES OF ACID-BASE BALANCE
Most acid-base disturbances result from
- disease or damage to organs (kidney, lungs, brain) whose normal function is necessary for acid-base homeostasis,
- disease which causes abnormally increased production of metabolic acids such that homeostatic mechanisms are overwhelmed
- medical intervention (e.g. mechanical ventilation, some drugs)
Arterial blood gases are the blood test used to identify and monitor acid-base disturbances. Three parameters measured during blood gas analysis, arterial blood pH (pH), partial pressure of carbon dioxide in arterial blood (pCO2(a)) and concentration of bicarbonate (HCO3 –) are of crucial importance (see Table I for reference (normal) range). Results of these three allow classification of acid-base disturbance to one of four etiological categories:
- Respiratory acidosis
- Respiratory alkalosis
- Metabolic acidosis
- Metabolic alkalosis
| Adults | Neonates | |
| pH | 7.35-7.45 | 7.30-7.40 |
| pCO2 (kPa) | 4.7-6.0 | 3.5-5.4 |
| Bicarbonate (mmol/L) | 22-28 | 15-25 |
TABLE I. Approximate reference (normal) ranges
To understand how the results of pH, pCO2(a) and bicarbonate are used to classify acid-base disturbances in this way, we must return to the Henderson-Hasselbalch equation
pH = 6.1 + log ([HCO3 –] / [H2CO3])
We measure pH and bicarbonate but not carbonic acid (H2CO3). However, there is a relationship between pCO2(a) and H2CO3 which allows restatement of the Henderson-Hasselbalch equation in terms of the three parameters (pH, pCO2(a) and bicarbonate) measured during blood gas analysis:
pH = 6.1 + log ([HCO3 –] / (pCO2(a) × 0.23))
By removing all constants from this equation, the relationship between the three measured parameters can be more simply stated:
pH ∝ [HCO3 –] / pCO2(a)
This relationship, crucial for an understanding of all that follows concerning acid base disturbances, states that arterial blood pH is proportional to the ratio of bicarbonate concentration to pCO2(a). It allows the following deductions:
- pH remains normal so long as the ratio [HCO3 –] : pCO2(a) remains normal
- pH increases (i.e. alkalosis occurs) if either [HCO3 –] increases or pCO2(a) decreases.
- pH decreases (i.e. acidosis occurs) if either [HCO3 –] decreases or pCO2(a) increases
- If both pCO2(a) and [HCO3 –] are increased by relatively the same amount, the ratio and therefore the pH are normal
- If both pCO2(a) and [HCO3 –] are decreased by relatively the same amount, the ratio and therefore the pH are normal.
Acid-base disturbances affect primarily either pCO2(a), in which case it is called a respiratory disturbance, or [HCO3 –], in which case it is called a non-respiratory or metabolic disturbance:
- If the primary disturbance is a raised pCO2(a) (which causes acidosis – see above), the condition is called respiratory acidosis
- If the primary disturbance is a reduced pCO2(a) (which causes alkalosis – see above), the condition is called respiratory alkalosis
- If the primary disturbance is associated with reduced bicarbonate (which results in acidosis – see above), the condition is called metabolic acidosis
- If the primary disturbance is associated with raised bicarbonate (which results in alkalosis – see above), the condition is called metabolic alkalosis
Causes of acid-base disturbances
Respiratory acidosis – (raised pCO2(a), reduced pH)
Respiratory acidosis is characterized by increased pCO2(a) due to inadequate alveolar ventilation (hypoventilation) and consequent reduced elimination of CO2 from the blood. Respiratory disease, such as bronchopneumonia, emphysema, asthma and chronic obstructive airways disease, may all be associated with hypoventilation sufficient to cause respiratory acidosis.
Some drugs (e.g. morphine and barbiturates) can cause respiratory acidosis by depressing the respiratory center in the brain. Damage or trauma to the chest wall and the musculature involved in the mechanics of respiration may reduce ventilation rate. This explains the respiratory acidosis that can complicate the course of diseases such as poliomyelitis, Guillain-Barre syndrome and recovery from severe chest trauma.
Respiratory alkalosis – (reduced pCO2(a), increased pH)
By contrast, respiratory alkalosis is characterized by decreased pCO2(a) due to excessive alveolar ventilation and resulting excessive elimination of CO2 from blood. Disease in which, due to reduced oxygen in blood (hypoxemia), the respiratory center is stimulated can result in respiratory alkalosis.
Examples here include severe anemia, pulmonary embolism and adult respiratory syndrome. Hyperventilation sufficient to cause respiratory alkalosis can be a feature of anxiety attacks and response to severe pain. One of the less welcome properties of salicylate (aspirin) is its stimulatory effect on the respiratory center. This effect accounts for the respiratory alkalosis that occurs following salicylate overdose. Finally, overenthusiastic mechanical ventilation can cause respiratory alkalosis.
Metabolic acidosis – (decreased HCO3 –, decreased pH)
Reduced bicarbonate is always a feature of metabolic acidosis. This occurs for one of two reasons: increased use of bicarbonate in buffering an abnormal acid load or increased losses of bicarbonate from the body. Diabetic ketoacidosis and lactic acidosis are two conditions characterized by overproduction of metabolic acids and consequent exhaustion of bicarbonate.
In the first case, abnormally high blood concentrations of keto-acids (b-hydroxybutyric acid and acetoacetic acid) reflect the severe metabolic derangements which result from insulin deficiency.
All cells produce lactic acid if they are deficient of oxygen, so increased lactic acid production and resulting metabolic acidosis occur in any condition in which oxygen delivery to the tissues is severely compromised.
Examples include cardiac arrest and any condition associated with hypovolemic shock (e.g. massive fluid loss). The liver plays a major role in removing the small amount of lactic acid that is produced during normal cell metabolism, so that lactic acidosis can be a feature of liver failure.
Abnormal loss of bicarbonate from the body can occur during severe diarrhea. If unchecked, this can lead to metabolic acidosis. Failure to regenerate bicarbonate and excrete hydrogen ions explains the metabolic acidosis that occurs in renal failure.
Metabolic alkalosis – (increased HCO3 – , increased pH)
Bicarbonate is always raised in metabolic alkalosis. Rarely, excessive administration of bicarbonate or ingestion of bicarbonate in antacid preparation can cause metabolic alkalosis, but this is usually transient. Abnormal loss of hydrogen ions from the body can be the primary problem. Bicarbonate which would otherwise be consumed in buffering these lost hydrogen ions consequently accumulates in blood. Gastric juice is acidic and gastric aspiration or any disease process in which gastric contents are lost from the body represents a loss of hydrogen ions.
The projectile vomiting of gastric juice, for example, explains the metabolic alkalosis that can occur in patients with pyloric stenosis. Severe potassium depletion can cause metabolic alkalosis due to the reciprocal relationship between hydrogen and potassium ions.
Compensation – a consequence of acid-base disturbance
It is vital for life that pH does not waiver too far from normal, and the body will always attempt to return an abnormal pH towards normal when acid-base balance is disturbed. Compensation is the name given to this life-preserving process. To understand compensation, it is important to recall that pH is governed by the ratio [HCO3 –] : pCO2(a). So long as the ratio is normal, pH will be normal.
Consider the patient with metabolic acidosis whose pH is low because bicarbonate [HCO3 –] is low. To compensate for the low [HCO3 –] and restore the all-important ratio towards normal the patient must lower his pCO2(a). Chemoreceptors in the respiratory center of the brain respond to a rising hydrogen ion concentration (low pH), causing increased ventilation (hyperventilation) and thereby increased elimination of carbon dioxide; the pCO2(a) falls and the ratio [HCO3 –] : pCO2(a) returns towards normal.
Compensation for metabolic alkalosis in which [HCO3 –] is high, by contrast, involves depression of respiration and thereby retention of carbon dioxide so that the pCO2(a) rises to match the increase in [HCO3 –]. However, depression of respiration has the unwelcome side effect of threatening adequate oxygenation of tissues. For this reason respiratory compensation of metabolic alkalosis is limited.
Primary disturbances of pCO2(a) (respiratory acidosis and alkalosis) are compensated for by renal adjustments of hydrogen ion excretion which result in changes in [HCO3 –] that compensate appropriately for primary change in pCO2(a). Thus the renal compensation for respiratory acidosis (raised pCO2(a)) involves increased reabsorption of bicarbonate, and renal compensation for respiratory alkalosis (reduced pCO2(a)) involves reduced bicarbonate reabsorption.
The concept of acid-base balance during compensation is conveyed visually in Fig. 2. Table II summarizes the blood gas results that characterize all four acid-base disturbances before and after compensation.
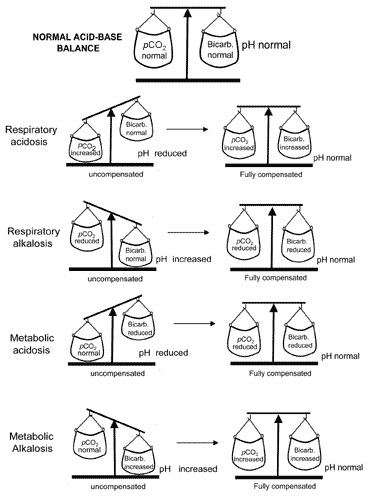
Fig. 2. The "acid-base balance" : compensation restores normal pH
| Primary disturbance | ||||
| Respiratory acidosis primary increase in pCO2 | Repiratory alkalosis primary decrease in pCO2 | Metabolic acidosis primary decrease in bicarb. | Metabolic alkalosis primary increase in bicarb. | |
| Some common causes | Emphysema COPD Pneumonia Depression of repiratory center | Hyper- ventilation Anxiety attacks Stimulation of brain respiratory center | Renal failure Diabetic ketoacidosis Circulatory failure - clinical shock (lactic acidosis) | Bicarbonate admini- stration Potassium depletion |
| Compen- satory mechanism | RENAL increase bicarbonate | RENAL decrease bicarbonate | RESPIRA- TORY decrease pCO2 | RESPIRA- TORY increase pCO2 but limited compen- sation in metabolic alkalosis |
| Initial blood gas results (uncompen- sated) | pH decreased pCO2 increased Bicarbonate normal | pH increased pCO2 decreased Bicarbonate normal | pH decreased pCO2 normal Bicarbonate decreased | pH increased pCO2 normal Bicarbonate increased |
| Blood gas results after partial compen- sation | pH decreased but closer to normal pCO2 inreased Bicarbonate increased | pH increased but closer to normal pCO2 decreased Bicarbonate marginally decreased | pH decreased but closer to normal pCO2 marginally decreased Bicarbonate decreased | Limited compen- sation in metabolic alkalosis |
| Blood gas results after full compen- sation | pH normal pCO2 increased Bicarbonate increased | pH normal pCO2 decreased Bicarbonate decreased | pH normal pCO2 decreased Bicarbonate decreased | Limited compen- sation in metabolic alkalosis |
Print friendly version of table, pdf.
TABLE II. Blood gas results in disturbances of acid-base balance
Respiratory compensation for a primary metabolic disturbance occurs much more quickly than metabolic (renal) compensation for a primary respiratory disturbance. In the second case, compensation occurs over days rather than hours.
If compensation results in return of pH to normal then the patient is said to be fully compensated. But in many cases the compensation returns pH towards normal without actually achieving normality; in such cases the patient is said to be partially compensated.
For reasons described above, metabolic alkalosis is very rarely fully compensated.
Mixed acid-base disturbances
It might be assumed from the above discussion that all patients with acid-base disturbance suffer from only one of the four categories of acid-base balance. This may well be the case, but in particular circumstances patients can present with more than one disturbance.
For example, consider the patient with a chronic lung disease such as emphysema who has a long-standing partially compensated respiratory acidosis. If this patient were also a diabetic who had not taken his normal insulin dose and as a result was in a state of diabetic ketoacidosis, blood gas results would reflect the combined effect of both respiratory acidosis and metabolic acidosis.
Such mixed acid-base disturbances are not infrequent and may be difficult to unravel on the basis of arterial blood gas results alone.
Summary
The maintenance of normal blood pH involves several organ systems and depends on circulatory integrity. It is not surprising then that disturbance of acid-base balance can complicate the course of widely diverse diseases as well as trauma to many parts of the body. The body has considerable power to preserve blood pH, and disturbances usually imply either severe chronic disease or acute critical illness.
The results of arterial blood gas analysis can identify acid–base disturbance and provide valuable information as to its cause.
Some suggested further reading
- Thomson WST, Adams JF, Cowan RA. Clinical acid-base balance. Oxford: Oxford Medical Publications 1997
- Harrison RA. Acid-base balance. Respir Care Clin N. America 1995; 1,1: 7-21
- Woodrow P. Arterial blood gas analysis. Nursing Standard 2004; 18,21: 45-52
- Sirker AA, Rhodes A, Gounds RM, Bennet ED. Acid-base physiology: the 'traditional' and the 'modern' approach. Anaesthesia 2002; 57: 348-56
Disclaimer
May contain information that is not supported by performance and intended use claims of Radiometer's products. See also Legal info.
miesnerlegiring76.blogspot.com
Source: https://acutecaretesting.org/en/articles/an-introduction-to-acidbase-balance-in-health-and-disease
0 Response to "The State of Balance or Normality That the Human Body Continuously Tries to Attain is Referred to as"
Post a Comment